INTRODUCTION
Developmental toxicity is any morphological or functional alteration caused by chemical or physical insult that interferes with normal growth, homeostasis, development, differentiation, and/or behavior. Teratology is a specialized area of embryology. It is the study of the etiology of abnormal development (the study of birth defects). Teratogens therefore are xenobiotics and other factors that cause malformations in the developing conceptus. Examples of teratogens may include (Fig. 1): pharmaceutic compounds, substances of abuse, hormones found in contraceptive agents, cigarette components, and heavy metals. Also included in this category are viral agents, altered metabolic states induced by stress, and nutrient deficiencies (e. g., folic acid deficiency).
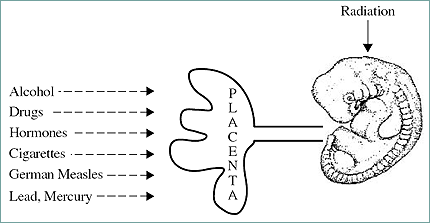
Fig. 13.1. Placenta and susceptibility to teratogens.
PRINCIPLES OF TERATOLOGY
James Wilson (in 1959) proposed six principles of teratology. A simplified version of these is as follows:
-
Susceptibility to teratogenesis depends on the embryo's genotype that interacts with adverse environmental factors (G x E interaction).
- The developmental stage of exposure to the conceptus determines the outcome.
- Teratogenic agents have specific mechanisms through which they exert there pathogenic effects.
-
The nature of the teratogenic compound of factor determines its access to the developing conceptus/tissue.
-
The four major categories of manifestations of altered development are death, malformation, growth retardation, and functional deficits.
-
The manifestations of the altered development increase with increasing dose (i. e., no effect to lethality).
When describing teratogens, one may think of three basic characteristics of teratogens:
- A given teratogen may be organ specific.
- It may be species specific.
- It can be dose specific.
Further discussion detailing these characteristics will be encountered later in the chapter when historical examples are addressed.
MAMMALIAN EMBRYOLOGY OVERVIEW
Prior to specific discussion of teratogenity, an embryology review is provided to facilitate the understanding of the principles and descriptions associated with the effects of teratogen exposure. This section will address the development from the zygote state to the attainment of the three germ layers (gastrula). Figure 13.2 diagrams the event leading to the development of the three-layered embryo, the gastrula.
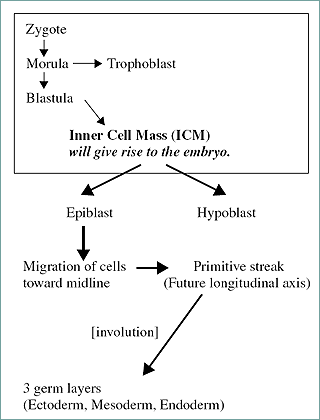
Figure 2. Development of the zygote to a three germ cell layered embryo.
Formation of the zygote marks the beginning of early embryonic development. The embryo proceeds from morula to the blastocyst while still within the zona pellucida. The aforementioned morula will give rise to the structure that attaches the early embryo to the uterus and feeds the embryo (trophoblast). Mammalian development is characterized by the formation of the blastocele-bearing embryo, the blastula (Fig. 3).
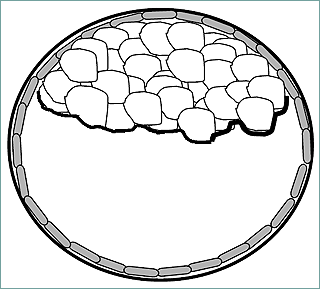
In the blastocyst stage (zona-intact) there is a distinct inner cell mass and an outer layer of trophectoderm cells.
Figure 3. Blastula containing the inner cell mass that gives rise to the embryo proper.
The blastula contains the mass of cells that will give rise to the actual embryo (conceptus). These cells, termed the inner cell mass (ICM), differentiate into ectoderm and endoderm prior to implantation. The ectoderm will eventually give rise to the epidermis and associated structures, the brain, and nervous system. The endoderm will give rise to glandular tissue such as the liver and pancreas and the linings of the gastrointestinal and respiratory tracts.
The inner cell mass gives rise to the epiblast (develops into ectoderm) and the hypoblast (develops into endoderm). Cells of the epiblast migrate toward the midline of the early embryo (Figg. 13.4 and 13.5).
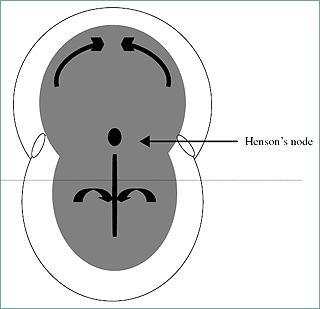
Figure 4. Dorsal view of the early embryo with the primitive evident. The horizontal dotted line represents sectioning that gives rise to the transverse view of the embryo in Figure 13.5. Curved arrows indicate migration of cells to the midline.
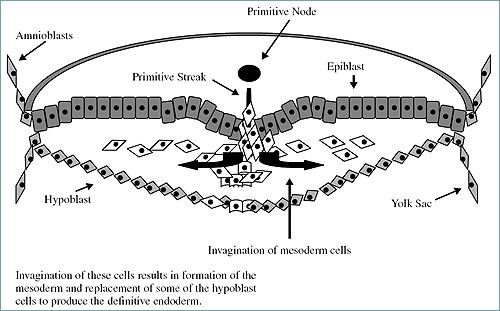
Figure 5. View of the transverse section of embryo in Figure 6. Note the migration of cells to form the mesoderm (involution).
The primitive streak is active proliferation of the cells with a loss of the basement membrane separating the epiblast and endoderm. The epiblast cells migrate and intermingle with the endoderm cells. The anterior end of the streak is defined by the Hensen's node. This node (also termed the primitive node) is associated with the organization of the developing embryo. The cellular migration (involution) leads to the creation of the third germ layer (the mesoderm). Somites are derived from the mesoderm. Figure 6 demonstrates the early stage embryo with visible somites and the succeeding embryonic to fetal stages.
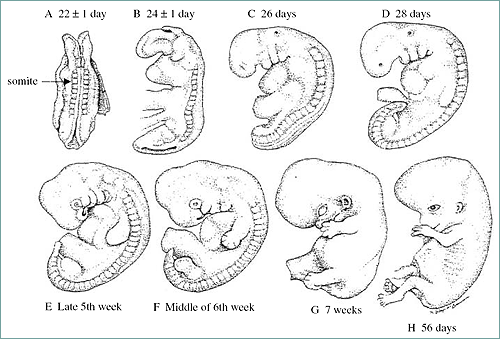
Somite: block-like mass of mesoderm alongside neural tube: forms vertebral column and segmental musculature.
Figure 6. Early stage human embryo with visible somites (represents a 22-day-old human embry or an 8-day-old mouse embryo).
Somites are blocklike masses of mesoderm alongside the neural tube. They will form the vertebral column and segmental musculature. They will also develop into the excretory system, gonads, and the outer covering of internal organs. Also formed from mesoderm are mesenchymal cells. These are loose migratory cells forming the dermis (inner skin layer), bones and cartilage, and circulatory system.
CRITICAL PERIODS
Major fetal outcomes depend on the stage of pregnancy affected, as there are critical periods for the development of fetal processes and organs. Although embryogenesis is complex involving cell migrations, proliferation, differentiation, and organogenesis, one may divide the developmental stages in to three large categories: pre-implantation, implantation to organogenesis, and the fetal to neonatal stage. The outcomes associated with exposure during these periods vary. This is not to say there are exceptions based on the type of exposure. However, the primary outcomes are as follows:
Stage of Exposure | Outcome(s) |
| Pre-implantation | Embryonic lethality |
| Implantation to time of organogenesis | Morphological defects |
| Fetal → neonatal stage | Functional disorders, growth retardation, carcinogenesis |
The sensitivity of the embryo to the induction of morphological defects is increased during the period of organogenesis. This period is essentially the time of the origination and development of the organs. The critical period graph (Fig. 7) demonstrates this point and definis the embryonic and fetal periods.
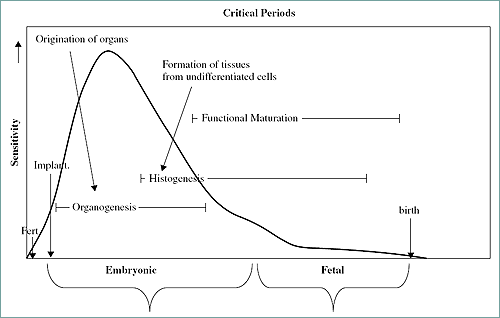
Figure 7. Critical periods graph including embryonic and fetal periods. Note the increased sensitivity to teratogenic events during organogenesis.
HISTORICAL TERATOGENS
Thalidomide
Thalidomide is a sedative-hypnotic drug used in Europe from 1957 to 1961. It was marketed for morning sickness, nausea, and insomnia. It went into general use and was widely prescribed in Europe, Australia, Asia, Africa, and the Americas. Women who had taken the drug from gestation days (GD) 35 to 50 gave birth to offspring suffering from a spectrum of different malformations, mainly amelia (absence of limbs) or phocomelia (sever shortening of limbs). Other malformations included: absence of the auricles with deafness, defects of the muscles of the eye and face, and malformations of the heart, bowel, uterus, and the gallbladder. The compound was withdrawn from the market in 1961 after about 10,000 cases had occured.
Accutane (Isotretinoin)
Accutane is a member of a family of drugs called retinoids, which are related to vitamin A. It is approved to treat serious forms of acne. These painful and disfiguring forms of acne do not respond to other acne treatments. Accutane is very effective, but its use is associated with a number of risks including birth defects. Exposure of pregnant women can lead to birth defects such as facial malformations, heart defects, and mental retardation.
Diethylstilbestrol (DES)
DES is a synthetic estrogen that inhibits ovulation by affecting release of pituitary gonadotropins. Some of its uses include treatment for hypogonadism, primary ovarian failure, and in some cases of prostate cancer. From 1940 to 1970, DES was used to help maintain pregnancy. In utero exposure to DES has been associated with abnormal development of the uterus. It has also been associated with certain types of tumors. Women who were exposed in utero often developed vaginal neoplasia, vaginal adenosis, and cervical erosion. Effects were not seen in offspring until they reached puberty. Clear cell carcinoma of the vagina is a type of adenocarcinoma found in young women who are exposed to diethylstilbestrol in utero. The reproductive organ of males can also be affected subsequent to in utero exposure. The outcomes include hypotrophic testes, poor semen volume and quality.
Alcohol
Fetal Alcohol Syndrome. Fetal alcohol syndrome (FAS) is a pattern of mental and physical defects that develops in some offspring when exposed to alcohol in utero. The first trimester is the most susceptible period. Some babies with alcohol-related birth defects, such as lower birth weight and body size and neurological impairments, do not have all of the classic FAS symptoms. These outcomes are often referred to as fetal alcohol effects (FAE). Currently there is not total agreement among medical scientists concerning the precise differences between FAE and FAS. In addition to growth retardation, the most common outcomes of fetal alcohol syndrome include psychomotor dysfunction and craniofacial anomalies.
The observed growth deficiences are associated with an inability of the baby to catch up due to a slower than normal rate of development. Other infrequent outcomes include skeletal malformations such as deformed ribs and sternum, scoliosis, malformed digits, and microcephaly. Distinctive facial anomalies have been associated with a diagnosis of fetal alcohol syndrome: small eye openings, epicanthal folds, failure of eyes to move in the same direction, short upturned nose, flat or absent groove between nose and upper lip, and thin upper lip. Visceral deformities may also be present: heart defects, genital malformations, kidney, and urinary defects.
A common concurrent manifestation of FAS include central nervous system defects. These include irregular arrangement of neurons and connective tissue. Mental retardation may also be present and associated with learning disabilities as well as difficulties in controlling body coordination.
"Non Chemical" Teratogens
Teratogens are not only xenobiotics. There may be other factors having the ability to cause malformations in the developing conceptus. Restraint stress in mice (12-hour restraint during early period of organogenesis) elicits axial skeletal defects (primarily supernumerary ribs). The Rubella virus (first reported in 1941, Austria) is associated with a number of fetal outcomes depending on the stage of development that the exposure occurs. Exposure during the first and second month of pregnancy was associated with heart and eye defects. Exposure during the third month was associated with hearing defects (and mental retardation in some cases).
TESTING PROTOCOLS
Formal testing guidelines were established after thalidomide disaster. In 1966 guidelines were established by the FDA: Guidelines for Reproduction Studies for Safety Evaluation of Drugs for Human Use. Since then (1994) new streamlined testing protocols have been developed with international acceptance. This newer approach, ICH (read below), relies on the investigator to determine the model to access reproductive/developmental toxicity. However, many scientists currently conduct and publish the FDA version of testing (i. e., segment studies). Therefore both (FDA & ICH) approaches will be discussed.
FDA Guidelines for Reproduction Studies for Safety Evaluation of Drugs for Human Use
Multigenerational Study. This approach involves the continuous exposure of a rodent species (usually mice). The parental animals are exposed shortly after weaning (30-40 days of age). At reproductive maturity, the animals are mated. The first generation is produced (F1). From these an F2 is produced and then subsequently an F3 generation. The effects of the test is monitored through each generation. The measured parameters include fertility, litter size, and neonatal viability. This is a time-consuming effort that usually takes about two years to complete.
Single-Generation Studies. Single-generation studies are short-term studies conducted in three segments:
Segment I: Evaluation of Fertility and Reproductive Performance. Male rodents are treated for 70 days (to expose for one spermatogenic cycle), and nonpregnant females for 14 days (to exposure for several estrous cycles). Treatment is continued in the females during mating, pregnancy, and lactation. Fifty percent of the females are killed and the fetuses are examined for presence of malformations. The other 50% are allowed to give birth. After weaning, these offspring are killed and necropsied.
Segment II: Assessment of Developmental Toxicity. This involves the treatment of pregnant females only during the period covering implantation through organogenesis (typically from gestational days 6 to 15 in mice with 18-day gestational periods). One day prior to birth, the animals are killed and fetuses examined for viability, body weight, and presence of malformation.
Segment III: Postnatal Evaluation. Pregnant animals are treated from the last trimester of pregnancy until weaning. Evaluated are parturition process, late fetal development, neonatal survival, and growth as well as presnce of any malformations.
International Conference of Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) — US FDA, 1994
As previously mentioned, new streamlined testing protocols with international acceptance have been developed. Below is a description of these guidelines as it relates to similarity to a particular segment-type study.
ICH 4.1.1: Fertility Assessment. This study duration shorter than segment I studies. Males are exposed for four weeks before mating and females two weeks before mating. Male reproductive organs are carefully evaluated: organ weights, histological analysis and sperm count, and mobility evaluation. For the females, fertility, litter size, and viability of conceptus are evaluated.
ICH 4.1.2: Postnatal Evaluation and Pregnancy State Susceptibility. This study protocol is similar to the segment III study. Maternal toxicity is evaluated by comparing the degree of toxicity of the nonpregnant female to that of the pregnant female. Postnatal viability and growth are also evaluated. Offspring are also evaluated to access functional development (i. e., presence of behavioral and reproductive deficits).
ICH 4.1.3: Assessment of Developmental Toxicity. This is almost identical to the segment II study protocol. Pregnant animals are exposed from implantation through organogenesis. The parameters measured in the segment II study are similar. However, the study is usually conducted using at least two species. More specifically, at least one rodent and one nonrodent species.
Alternative Test Methods
A number of alternative test methods have been developed to reduce the number of whole animals used in studies and/or obtain more rapid information concerning the potential of a compound to be a reproductive/developmental toxicant. Validation of many of the methods has been problematic, since they do not address the contribution of maternal factors or multiorgan contributions to outcomes. Some of these alternative methods include the use of cell or embryo culture. For example, the micromass culture involves the use of limb bud cells from rat embryos grown in micromass culture for five days. The processes of differentiation and cell proliferation are assessed. In the Chernoff/Kavlock Assay, pregnant rodents are exposed during organogenesis and allowed to deliver. Postnatal growth, viability, and gross morphology of litters are recorded (detailed skeletal evaluations are not performed). Other alternative tests involve the use of nontraditional test species such as Xenopus embryos (FETAX) and Hydra. Xenopus embryos are exposed for 96 hours and then evaluated for morphological defects, viability, and growth. The cells of Hydra aggregate to form articifial embryos. The dose response in these "embryos" is compared to that of the adult Hydra.
CONCLUSIONS
Understanding the mechanisms of the induction of birth defects is key to determine how to prevent these effects. Further, increasing the accuracy of experimental animal extrapolation will aid in the interpretation of experimental data in order to more accurately determine the risk of a given compound to elicit birth defects in humans.
